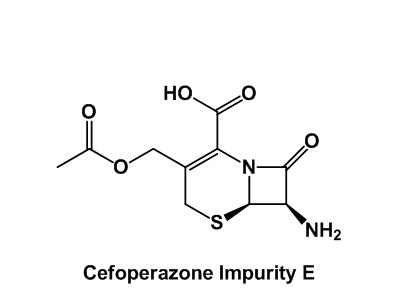
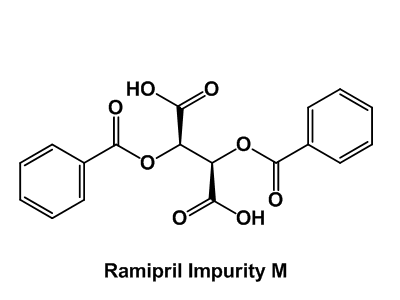
Impurity Reference Standards
Unidentified, potentially toxic impurities are health hazards. To increase the safety of drug therapy, impurities should be recognized and their content in API’s must be managed according to ICH and FDA guidelines. Enamine has widely documented scientific expertise in organic synthesis that has already helped us to synthesize a large number of previously unidentified impurities.
Our entire catalog counts now over 400 impurity reference standards. Their detailed certificates of analysis include clear-cut identity and purity information supported by NMR, HPLC/MS and/or GCMS data.
Download XLSX file
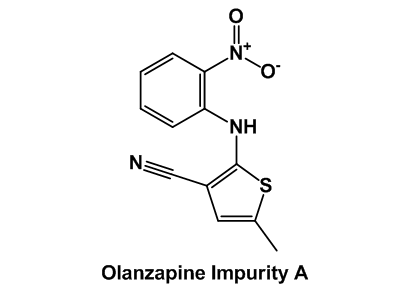
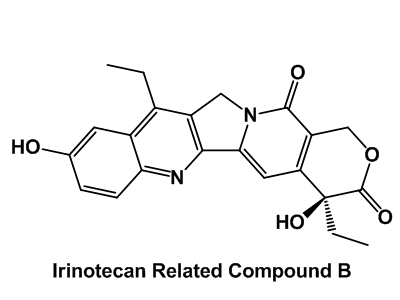
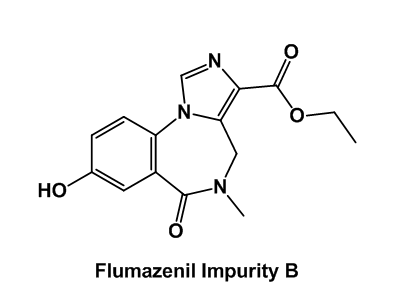
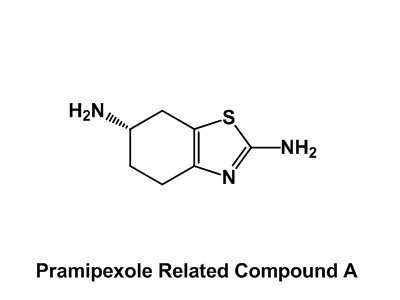
Drug impurity profiling
Enamine provides services in the analysis of API’s to identify impurities. We investigate all steps in the production process assessing any compound or solvent involved in it that could possibly be responsible for the formation of impurities or degradation products. This comprehensive analysis allows a reliable prediction of the structure of the unknown impurities, identification of their generation mechanism, and subsequently strategic impurity management.
Custom Synthesis
Our strong point and competitive advantage are in the design of synthesis routes. We don’t require documented synthesis procedure to produce your compound of interest. In most of the cases, we can propose a realistic synthesis scheme from scratch and successfully realize it producing a desirable amount of compound (from mg to gram scale) in the requested purity.
