Safe alternative to toxic isocyanates
Enamine Company presents new building blocks to our valued customers. They can be used as good precursors for the preparation of unsymmetrical ureas. We have developed and synthesized a set of compounds of general formula:
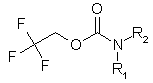
These compounds can be used as stable substitutes for hardly accessible and moisture sensitive aliphatic isocyanates. (Aguirre I. de; Collot J.; Bull.Soc.Chim.Belg.; 98; 1; 1989; 19-30).
These reagents are used for the synthesis of urea derivatives. Thus, trifluoroethyl carbamates react readily and selectively with primary and secondary amines producing unsymmetrical ureas and trifluoroethanol which can be easily removed. (Matsumura Y., Satoh Y., Onomura O., Maki T.; J. Org. Chem.; 65; 5; 2000; 1549 - 1551)
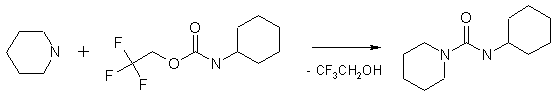
The method is a powerful tool for the introduction of ureidic moiety in the molecules. Isolation of the targeted ureas is very simple so the process can be automated. The trifluoroethyl carbamates are less hazardous compared to isocyanates and therefore safer to use in laboratory practice.
Original procedure for preparation of unsymmetrical ureas from trifluoroethyl carbamates has been developed at Enamine. It is more facile than original procedure described by Matsumura et al and can be utilized for combinatorial synthesis. Details of the newly developed procedure will be published shortly. We can provide additional information on this procedure to our customers upon purchase of trifluoroethyl carbamates.
This procedure has been widely exploited by Enamine to produce druglike ureas for our Screening Collection.
Additionally, various trifluoroethyl carbamates could be synthesized upon customers' request.
