Phenyl(trifluoromethyl)mercury
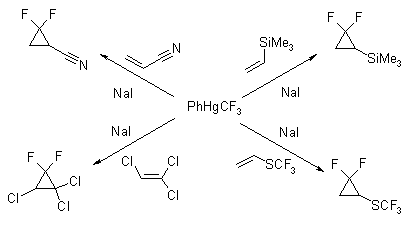
Current reagent is an effective and facile generator of difluorocarbene. Phenyl(trifluoromethyl)mercury is a stable, crystalline solid which releases CF2 under mild, nonbasic reaction conditions. The compound is hydrolytically stable and can be handled under standard laboratory conditions. The reaction of PhHgCF3 and 3 molar equiv of anhydrous sodium iodide in benzene medium in the presence of olefins serves excellently in the synthesis of gem-difluorocyclopropanes. High products yields could be obtained in reaction times of about 15hr at 80-85°C.
Few examples are given on Scheme.
References: Seyferth D., Hopper S. P. // J. Org. Chem., 1972, 37, p. 4070-4075
Haas A., Hinsken W. // J. Fluorine Chem., 1985, 28, p. 303-318
The reaction is stereospecific producing cyclopropanes with desired stereochemistry from the corresponding cis- or trans- alkenes.

Realizing value of the reagent we manufactured we have to offer it to our customers. To order material, receive technical information or suggest potential new products, please contact Enamine staff.
