Conformationally rigid amino acids
"The α-amino acids, molecules of which have restricted conformational flexibility, are widely used in design of peptidomimetics, peptide models, and in systematic search for biologically active compounds. Among these amino acids, a distinct class of compounds can be highlighted, namely - conformationally rigid amino acids (CRA). Certain torsion angles, which describe the conformation of a polypeptide chain at the CRA, are "fixed" that allows predicting and controlling it to some extent. Many structural studies show that the CRA residues can dictate certain conformation of the peptide chain around them, consequently, they can stabilize or destabilize certain peptide secondary structure elements.
"The α-amino acids, molecules of which have restricted conformational flexibility, are widely used in design of peptidomimetics, peptide models, and in systematic search for biologically active compounds. Among these amino acids, a distinct class of compounds can be highlighted, namely - conformationally rigid amino acids (CRA). Certain torsion angles, which describe the conformation of a polypeptide chain at the CRA, are "fixed" that allows predicting and controlling it to some extent. Many structural studies show that the CRA residues can dictate certain conformation of the peptide chain around them, consequently, they can stabilize or destabilize certain peptide secondary structure elements. Hence, the incorporation of the CRA in peptidomimetics and peptide models is a powerful tool for controlling their conformation and, to some extent, biological activity. In other words, CRA using as building blocks for peptidomimetic synthesis, gives better chances to find an efficient drug candidate."
Russ. Chem. Rev., 73 (2004), Nr 8, 785
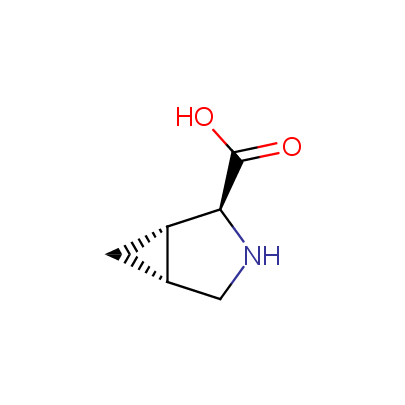
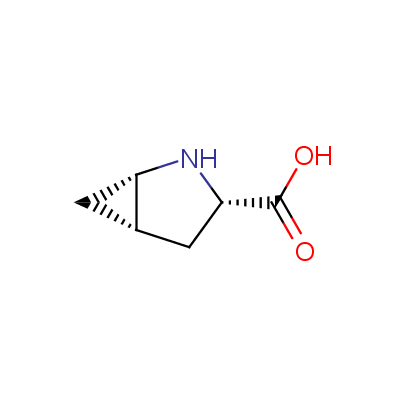
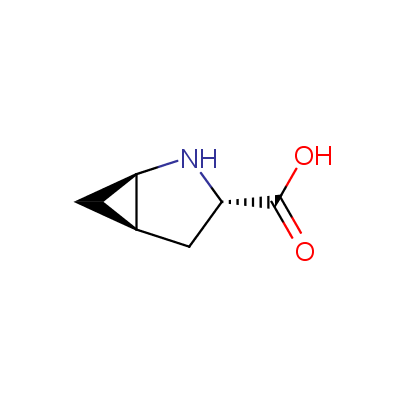
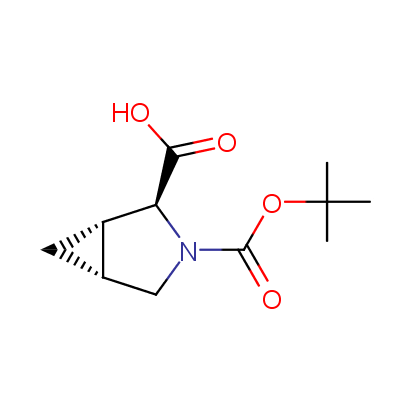
We offer a set of conformationally rigid proline analogues. This amino acid is frequently found in peptide β-turns, which play an important role in peptide receptor recognition and antigen determination (J. Mol. Biol. 1988, 203, 221-232). It is highly probable that the bioisosteric replacement of proline analogues for proline in the known biologically active peptides/drugs may lead to new potential drug candidates.
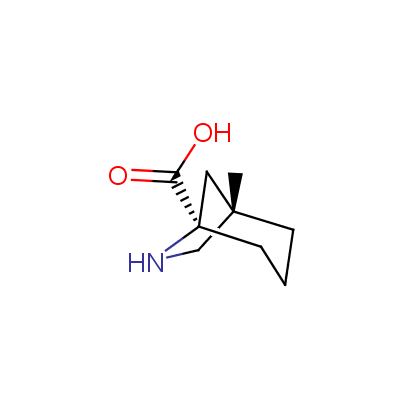
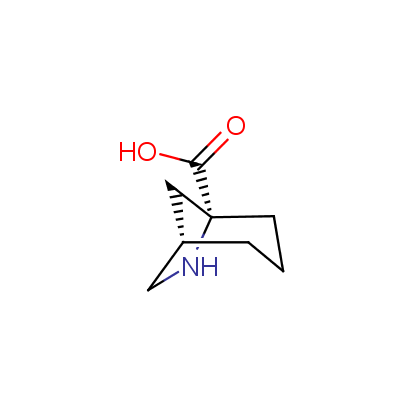
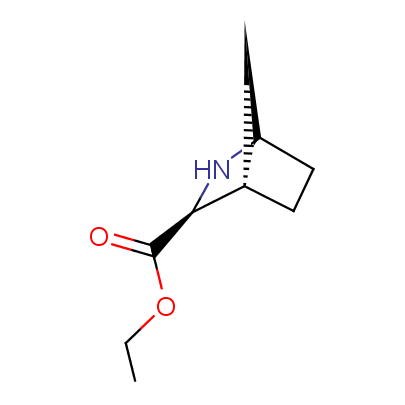
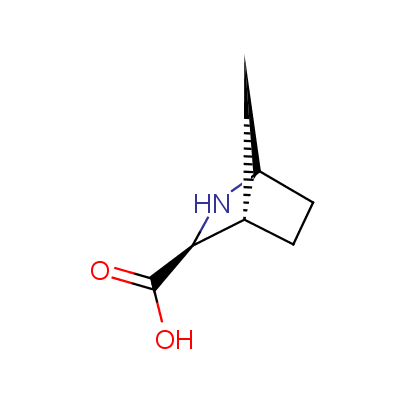
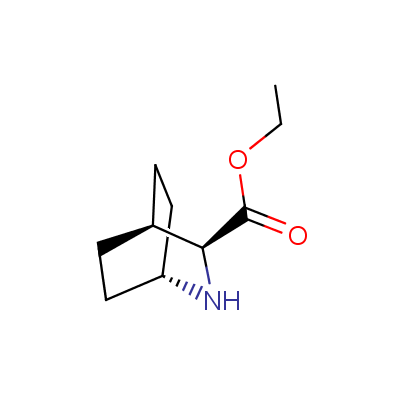
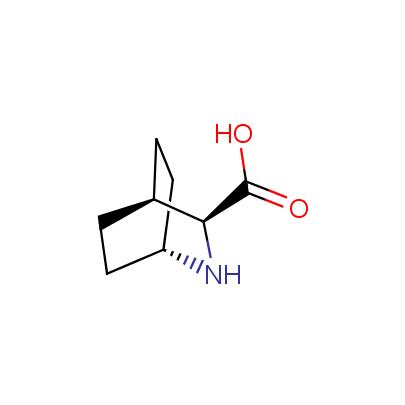
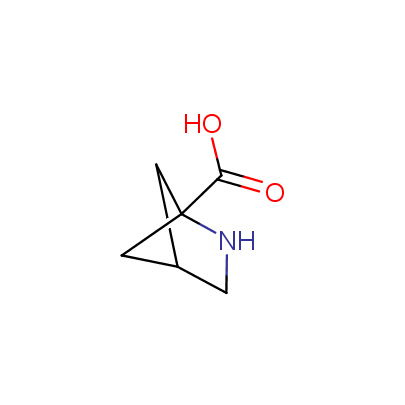
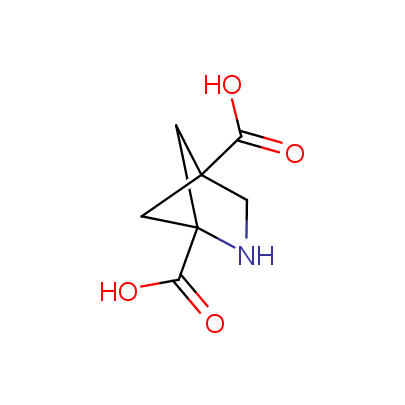
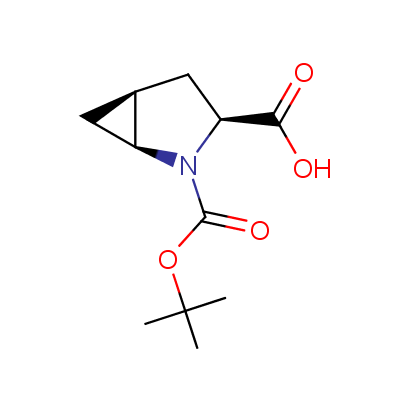
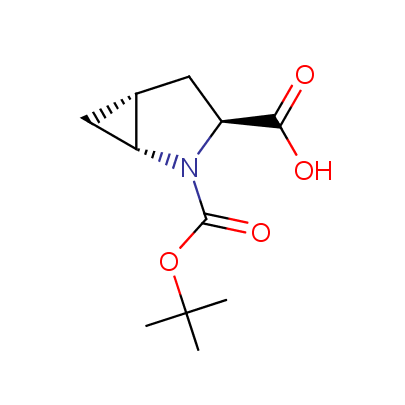
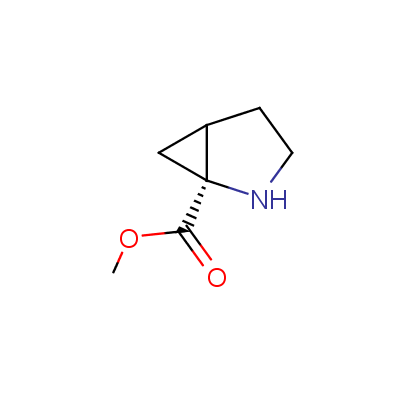
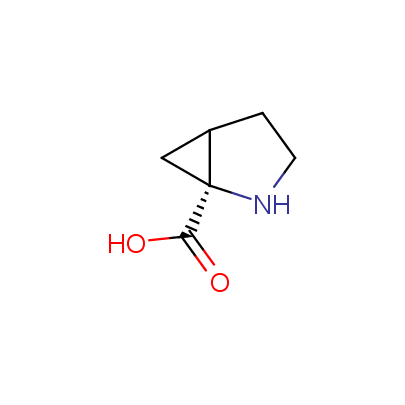
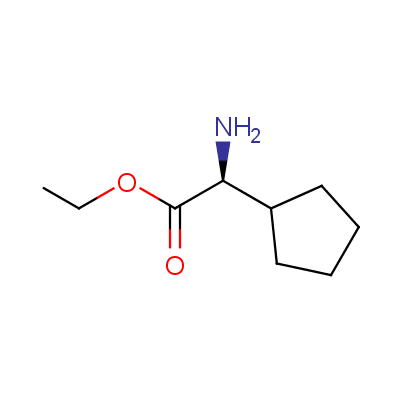
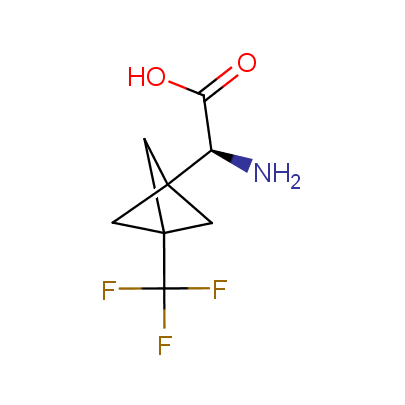
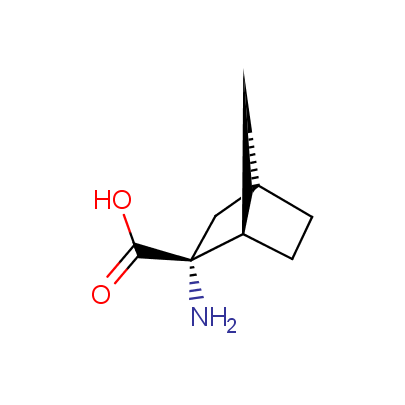
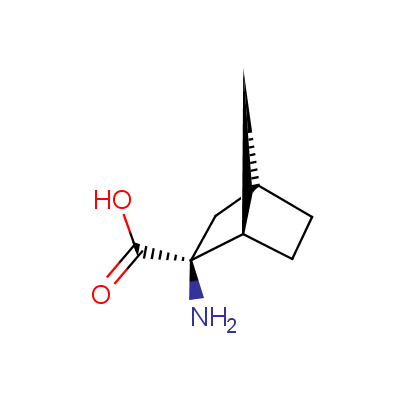
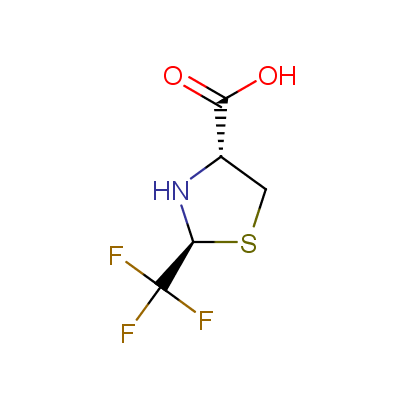
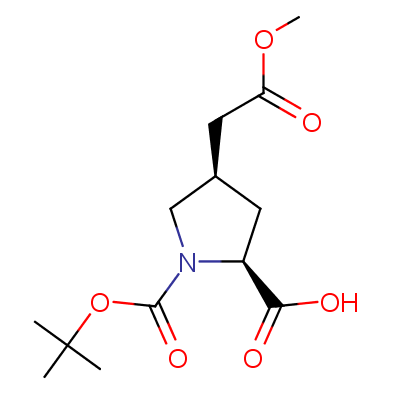
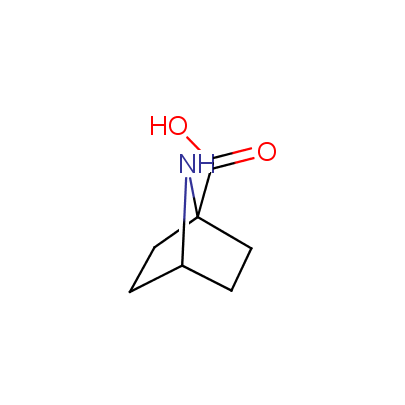
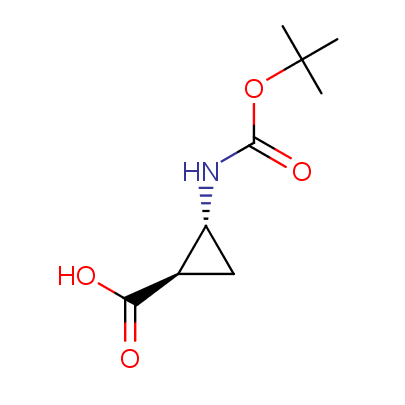
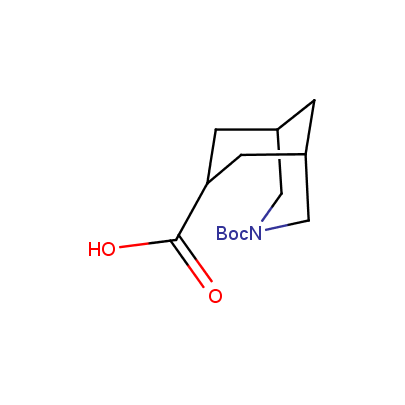
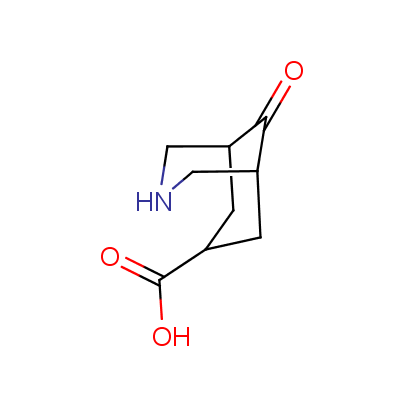
The table below shows the structural formula of our conformationally rigid amino acids. All products are >96% chemically pure, and >90% optically pure (if applicable).
Type I: Unnatural α-amino acids
Type II: Unnatural β- and ω- amino acids
Successful examples of the peptidomimetic drug design using conformationally rigid a-amino acids (highlighted in red) are illustrated below:
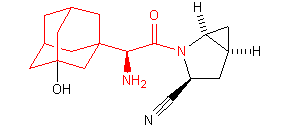
Saxagliptin (Bristol-Myers Squibb and AstraZeneca)
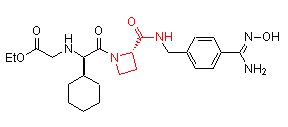
Ximelagatran (AstraZeneca)
Nature Reviews/Drug Discovery, 3 (2004), 649
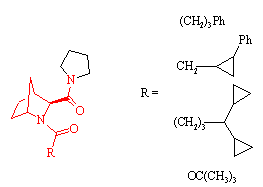
Prolylendopeptidase inhibitors
J. Med. Chem., 39, 2379 (1996)
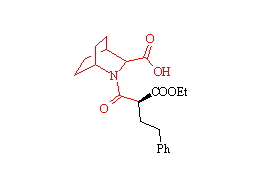
Zabicipril
Tetrahedron Lett., 33, 7369 (1992)