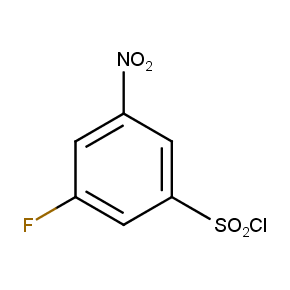
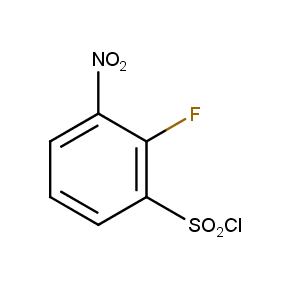Isomeric Fluoronitrobenzenesulfonyl Chlorides
The synthesis of functional organic compounds for different applications largely relies on laborious approaches involving an extensive usage of protecting groups. Nowadays chemoselective routes to the construction of complex molecules are becoming a good alternative because they shorten the length of multistep syntheses by excluding protecting groups and, as a consequence, lead to increased overall yields.
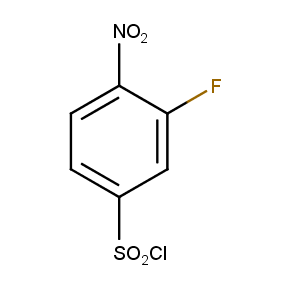
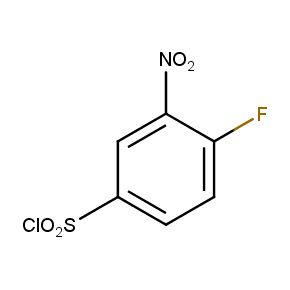
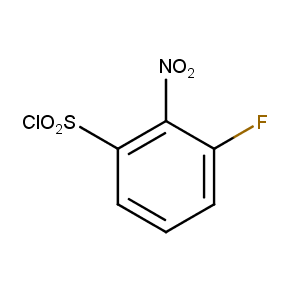
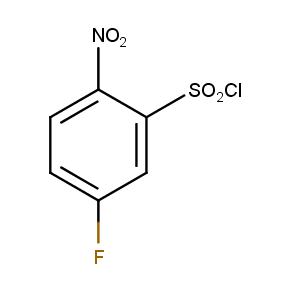
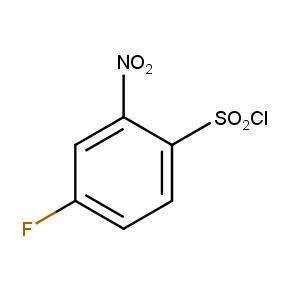
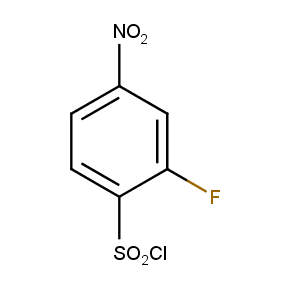
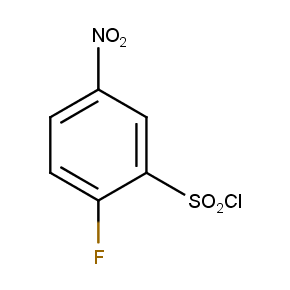
The ten constitutionally isomeric fluoronitrobenzenesulfonyl chlorides shown in Figure 1 contain three selectively addressable functional groups which could be employed in numerous synthetic transformations involving sulfonylation, arylation, and reduction with the follow up chemistry. For example, stepwise derivatizations of some fluoronitrobenzenesulfonyl chlorides were successfully used in syntheses of drug-like compounds. Given the fact that the fluoronitrobenzenesulfonyl chlorides are isomers they can be used in preparation of isosteric sets of biologically potent compounds. Synthesis of new functional branched macromolecular assemblies such as dendrimers and hyperbranched polymers is another way to use these compounds. For example, the fluoronitrobenzenesulfonyl chlorides can be employed as core units for attaching dendrons of up to three different types or, alternatively, they can be used as branching units in repetitive steps of reduction-sulfonylation in divergent synthesis of functionalized dendrimers.
Chemists of Enamine have developed synthetic routes to five hitherto unknown isomers of fluoronitrobenzenesulfonyl chloride (Figure 1). Now we are offering most of described isomeric fluoronitrobenzenesulfonyl chlorides from stock. Please contact us for information on availability and pricing of listed fluoronitrobenzenesulfonyl chlorides.
Figure 1. The full set of constitutionally isomeric fluoronitrobenzenesulfonyl chlorides offered by Enamine. The preparation of compounds EN300-55293, EN300-55294, EN300-10260, EN300-43690, and EN300-35187 was reported in the literature. Compound EN300-43690 was obtained in our laboratories by a new one-step procedure instead of the published five-step method. Compounds EN300-45567, EN400-16449, EN300-09832, EN300-43514, and EN300-49805 were synthesized in our laboratories for the first time by our own procedures.