Cyclic Sulfones
This issue of Enamine Product Focus represents a family of Building Blocks possessing sulfone moiety as a part of a cyclic unit. All the building blocks processing cyclic sulfone moiety have some common features that make the design of potential drug candidates particularly efficient.
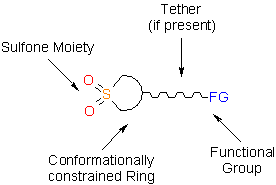
- Sulfone moiety, a strong H-bond acceptor which is considered as carbonyl group bioisostere, enforces interaction of the molecule with potential biological target.
- Conformationally constrained five-, six- or seven-membered ring fixes the functional group responsible for linking the sulfolane part to other fragments of the molecule, thus reducing entropy of binding of the latter with potential biological target. In some cases, an additional flexible or conformationally constrained tether is also present.
- Functional group allows tethering cyclic sulfone building block to other fragments of the potential drug candidate molecule; moreover, it is often also capable of interaction with potential biological target through H-bonding.
- A distance between two H-bonding units mentioned above (2–5 bonds) is relevant for the most efficient interaction with different biomolecules.
Several examples of drugs and drug candidates possessing cyclic sulfone moiety that have reached pre-clinical testing include antiglaucoma agent Dorzolamide, antitrypanosomal drug Nifurtimox (Lampit®), compounds with antiviral or antipsychotic activity.
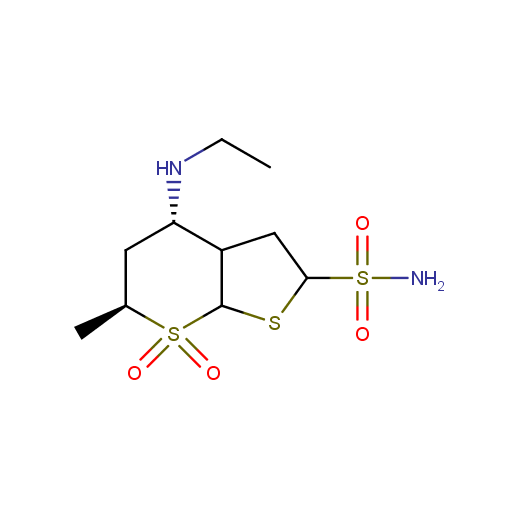
Antiglaugoma
(Dorzolamide)
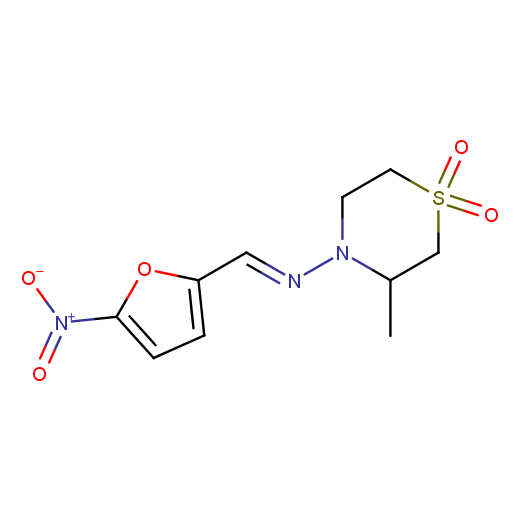
Antitrypanosomal
(Nifurtimox)
Antipsychotic
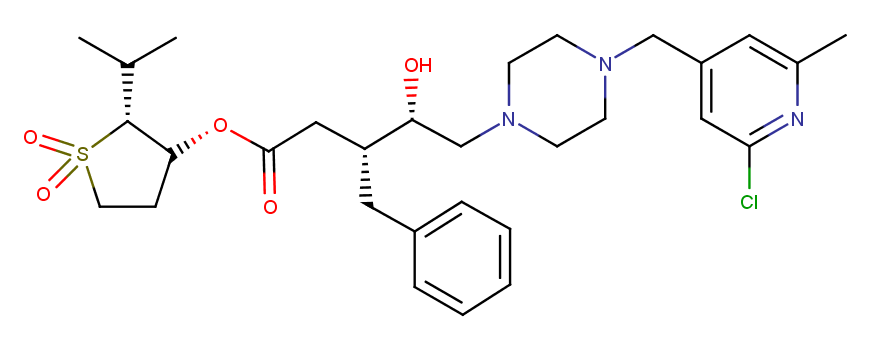
HIV I Protease Inhibitor
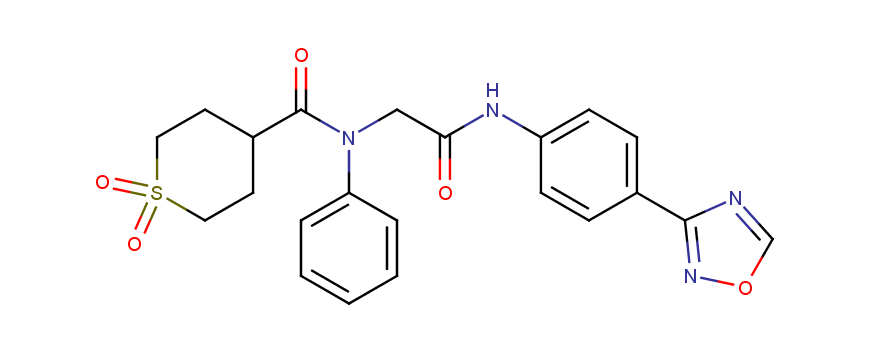
Antiviral
Enamine offers several subsets of building blocks possessing cyclic sulfone moiety. Most of them are derivatives of five-membered heterocycle Sulfolane. Apart from a set of building blocks containing sulfolane unit, cyclic sulfones with six- and seven-membered rings (see below) are also offered. The procedures developed for the synthesis of cyclic sulphones allow obtaining a large diversity of these building blocks at 1–10 g scale; novel compounds of the requested structure can be obtained in 4–8 weeks. Scale-up to 1 kg can be performed upon request.
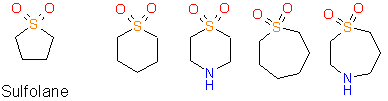
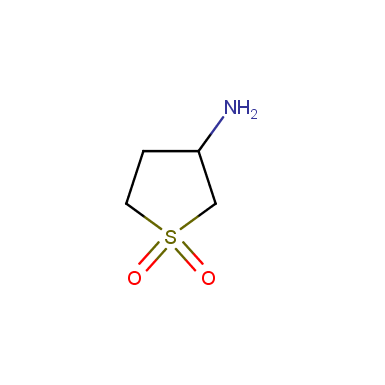
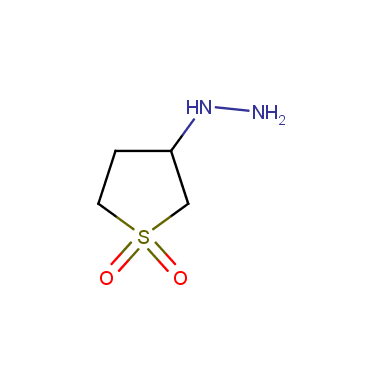
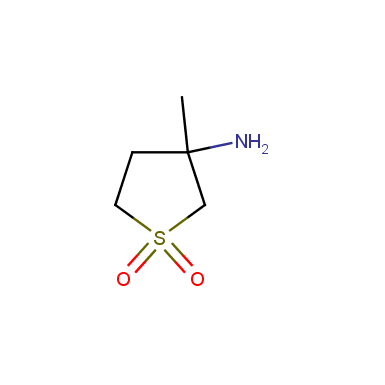
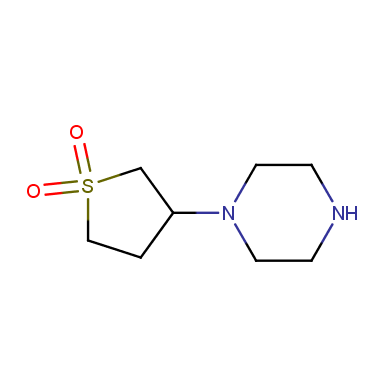
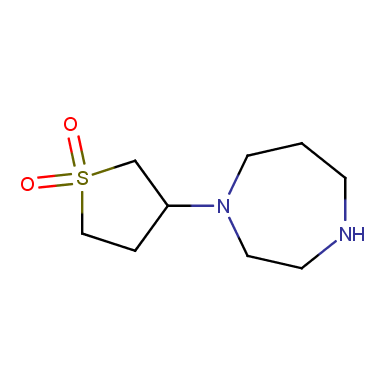
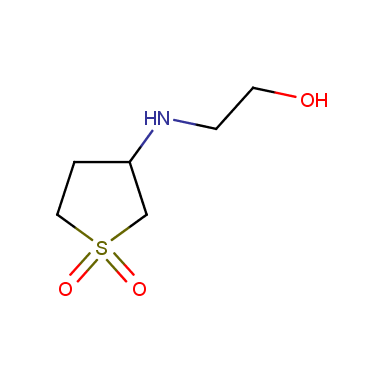
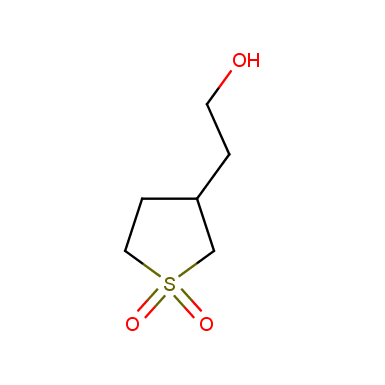
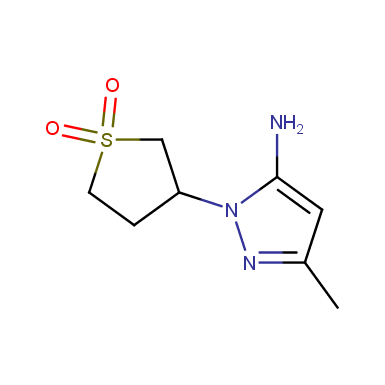
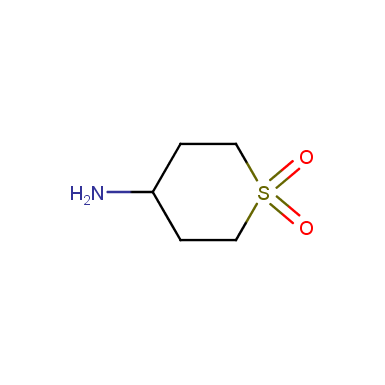
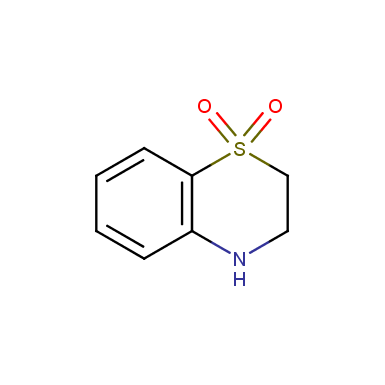
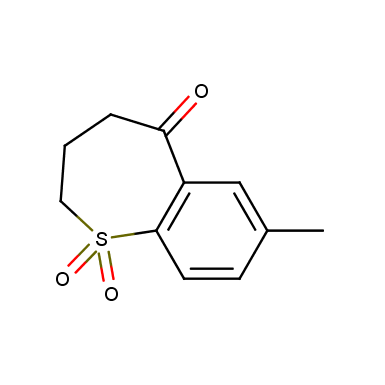
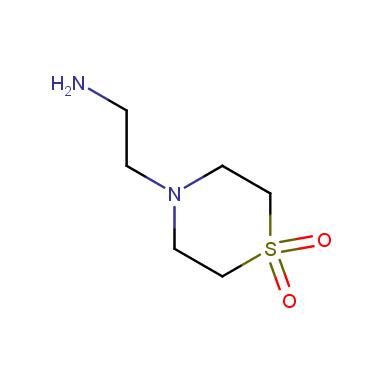
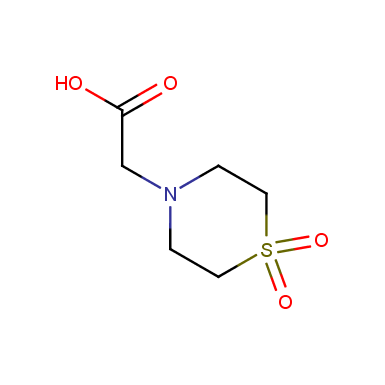
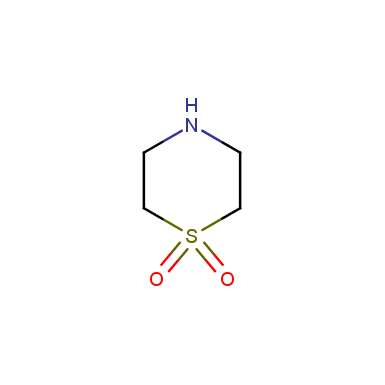
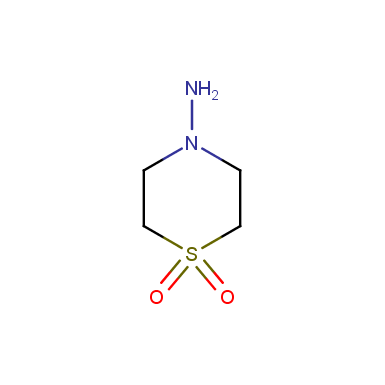
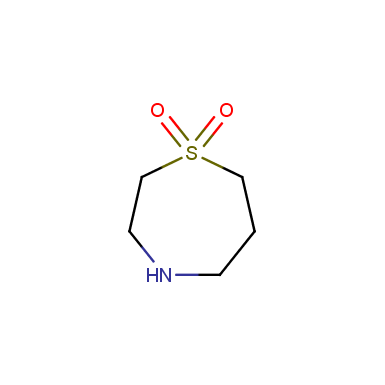
Some examples of cyclic sulfones are shown below.
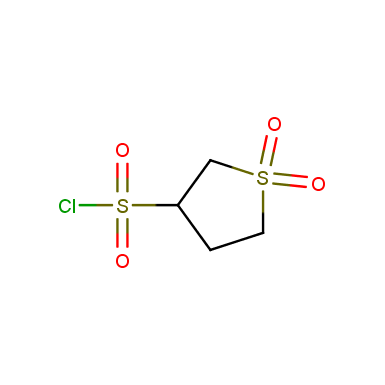
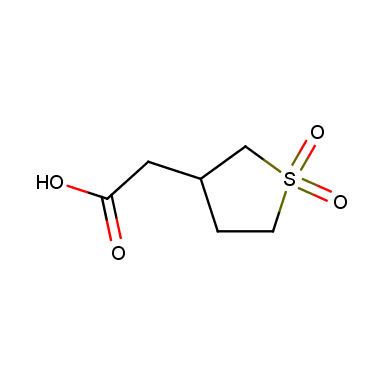
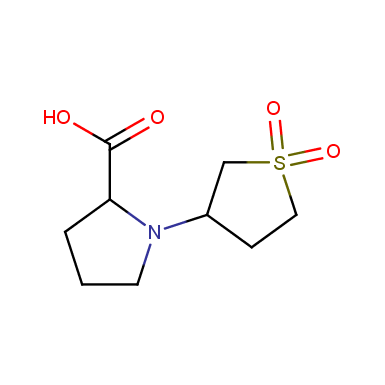
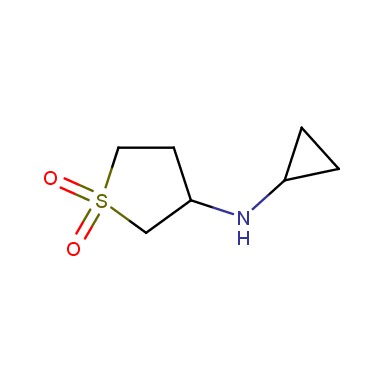
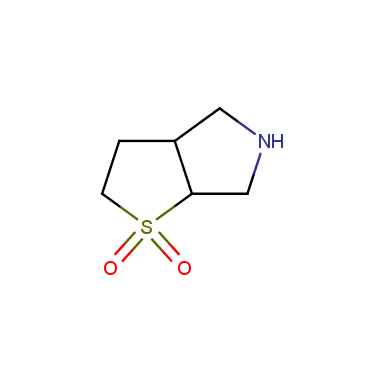
Sulfolane derivatives
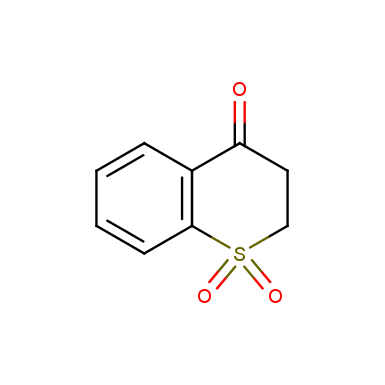
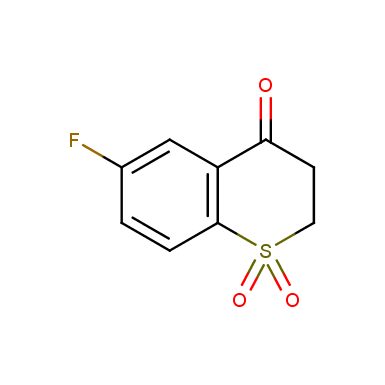
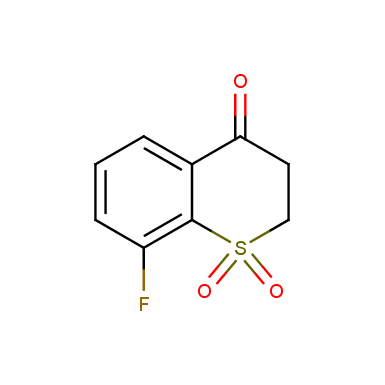
Other cyclic sulfones