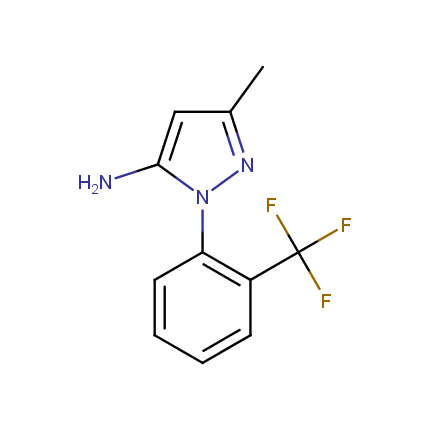
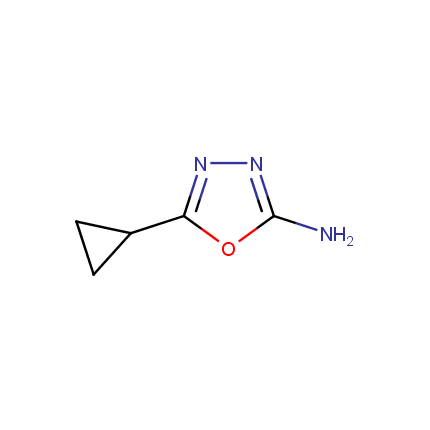
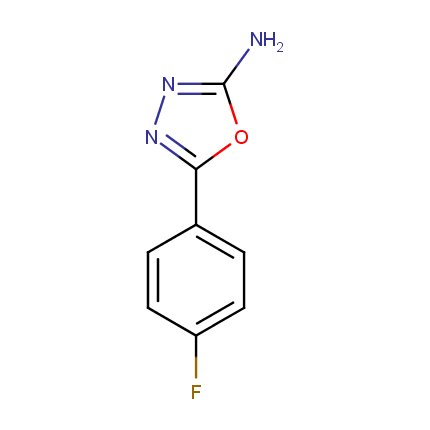
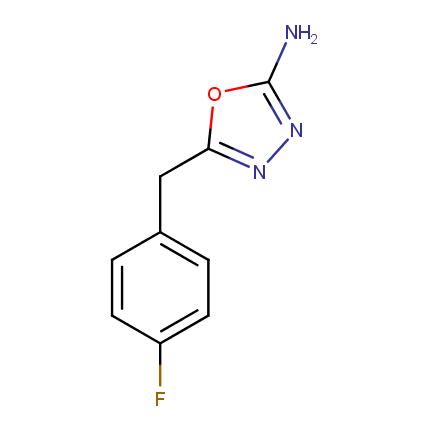
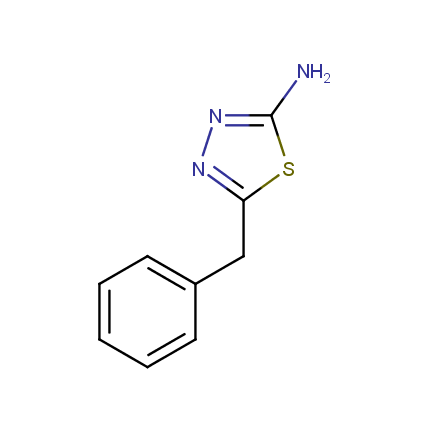
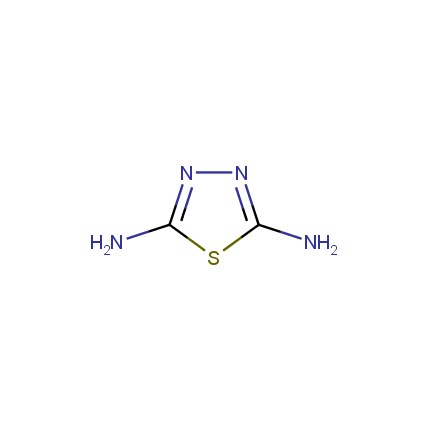
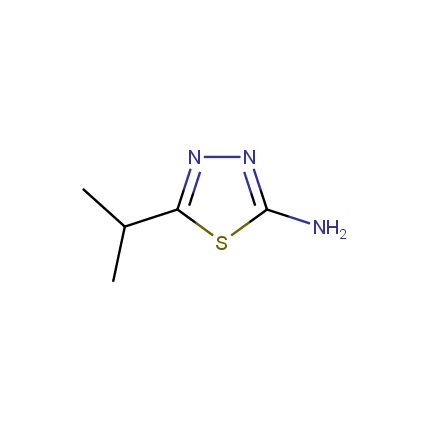
Amino Azoles
The intrinsic role of heterocyclic compounds in medicinal chemistry is now undoubtful as more than 90% of new drugs contain heterocycles. Compounds possessing heterocyclic cores have several advantages that make their use in drug design particulary effective.
- Aromatic rings are extremely rigid in conformation thus defining spatial orientation of attached functional groups and reducing entropy of binding of the molecule with potential biological target.
- In most cases, hetero atoms exhibit properties of strong hydrogen bond donors/acceptors enforcing interaction of the molecule with its biological target.
- The chemistry of heterocyclic compounds is undoubtedly the most explored area of organic chemistry. The synthetic methods for the construction of heterocycles are well-established, simple, highly efficient and easy-scalable.
- Heterocycles occupy an overwhelming majority of the available chemical space thus being the most diverse class of organic compounds. Therefore they represent the substantial possibilities for the design of novel scaffolds that are yet not protected by patents.
The diversity of heterocyclic compounds relies on the large variety of building blocks available for their synthesis. This issue of Enamine Product Focus represents a family of Amino Azole Building Blocks. Despite the considerable part of amino azoles exhibit chemical behavior which is common for other aromatic amines (e. g. undergo acylation reactions), considerable mutual influence of amino and azole functions leads to peculiar chemical properties. In particular, nucleophilicity of azole ring atoms is enhanced whereas that of the amino group is somewhat reduced. Thus the reactions of amino azoles with various bis-electrophiles allow fusion of additional heterocyclic ring(s) to the azole moiety.
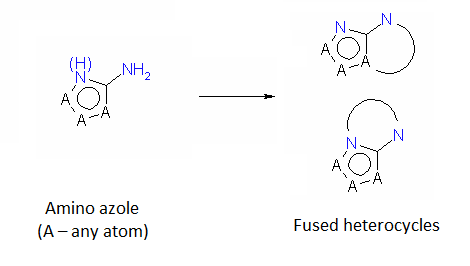
An important subtype of fused heterocycles that can be obtained from amino azoles is represented by Purines that are widespread in the nature (e. g., Adenine, Guanine and Caffeine).
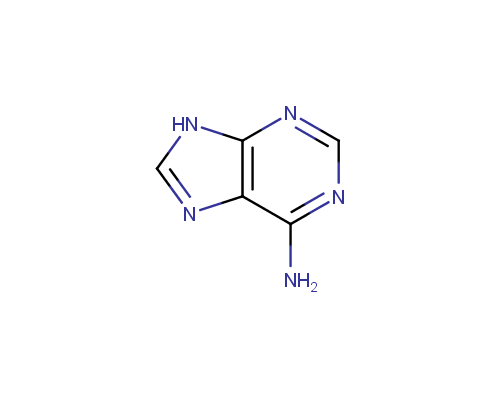
Adenine
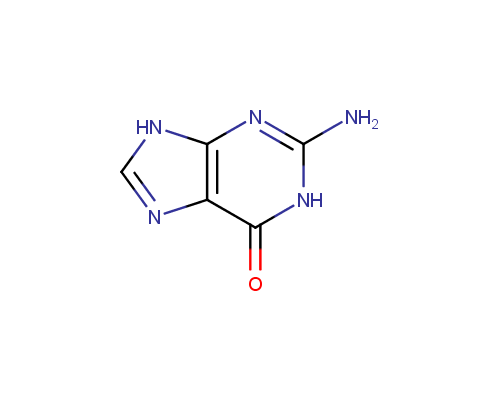
Guanine
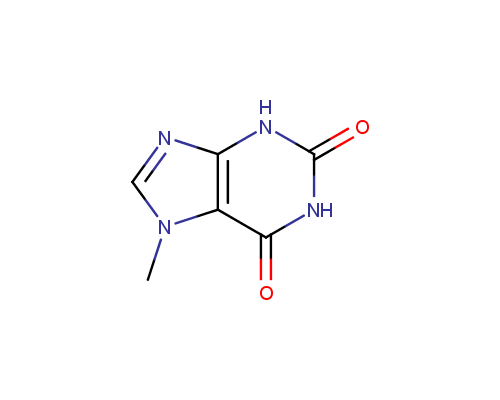
Caffeine
Examples of the drugs containing fused heterocycles that can be obtained from amino azoles include anti-hyperuricemic agent Allopurinol (also used for treatment of ischaemic reperfusion injury, kidney stones with an uric acid component and protozoal infections), immunosuppressant Azatioprine (Imuran® or Azasan®), Temozolomide (Temodar®) used for the treatment of Grade IV astrocytoma, anticancer chemoteurapeutic drug Deoxycoformycin (Pentostatin®) and sedative Zaleplon (Sonata®) used for insomnia treatment.
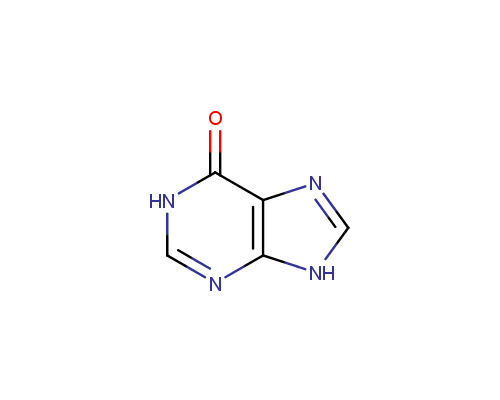
Allopurinol
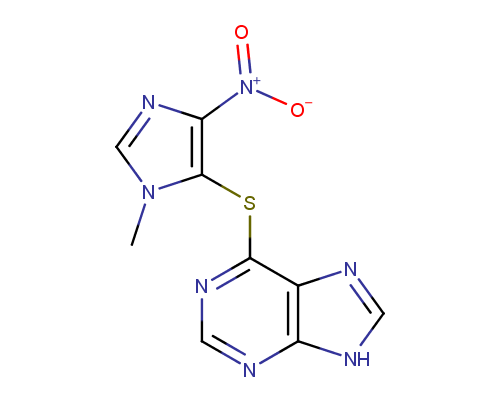
Azatioprine
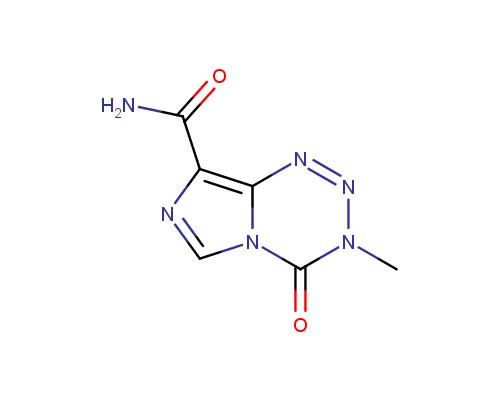
Temozolomide
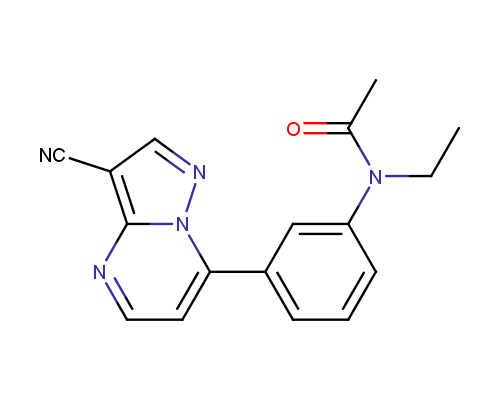
Zaleplon
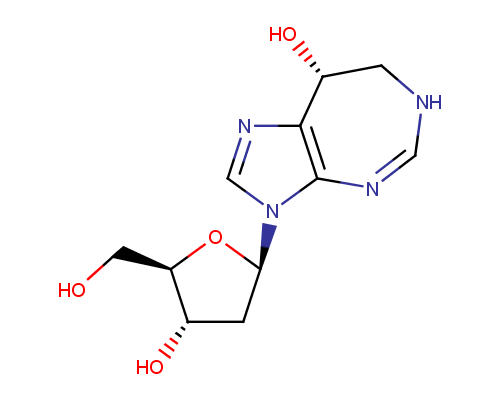
Deoxycoformycin
Amino azoles themselves are also rather often used as scaffolds in drug design. In particular, some of them can be considered as bioisosteres for primary amide group C(O)NH2. The drugs containing amino azole moiety are represented by Pramipexole (Mirapexin®), a medication indicated for treating Parkinson's disease and restless legs syndrome; Acetazolamide (Diamox®) which is used to treat glaucoma, epileptic seizures, benign intracranial hypertension, altitude sickness, cystinuria, and dural ectasia; Riluzole, a drug used to treat amyotrophic lateral sclerosis, carbonic anhydrase inhibitor; fourth generation cefallosporine antibiotic Cefozopran; antiprotozoal agent Nitazoxanide (Alinia®), and tyrosine kinases inhibitor Dasatinib (Sprycel®) approved for use in patients with chronic myelogenous leukemia Philadelphia chromosome-positive acute lymphoblastic leukemia.
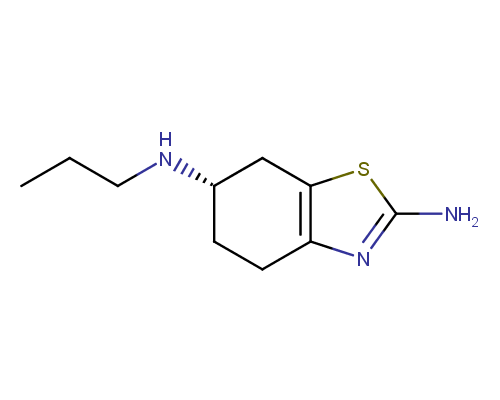
Pramipexole
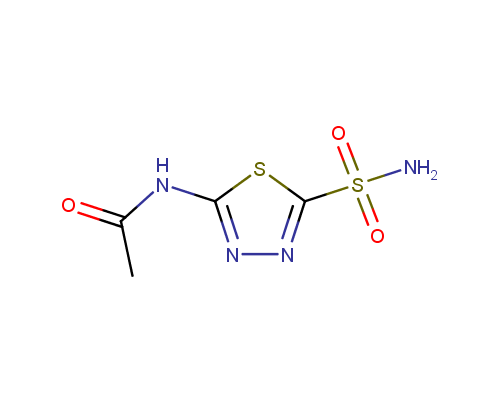
Acetazolamide

Riluzole
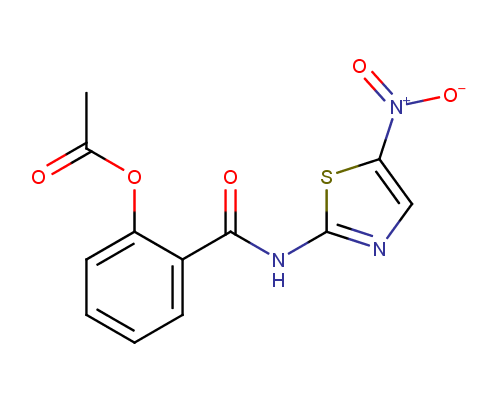
Nitazoxanide
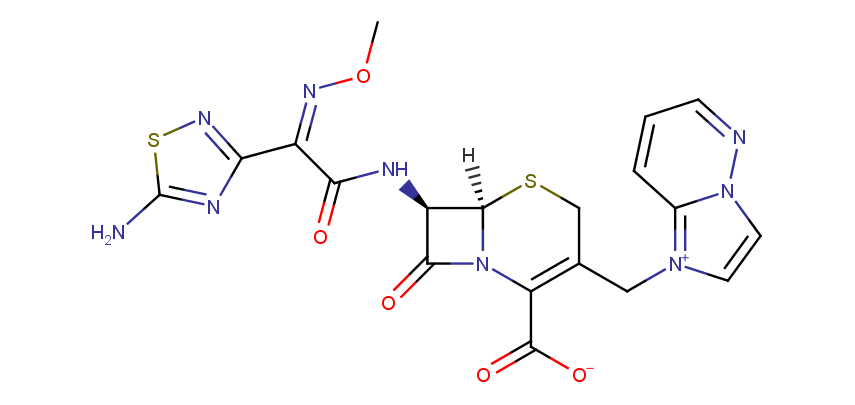
Cefozopran
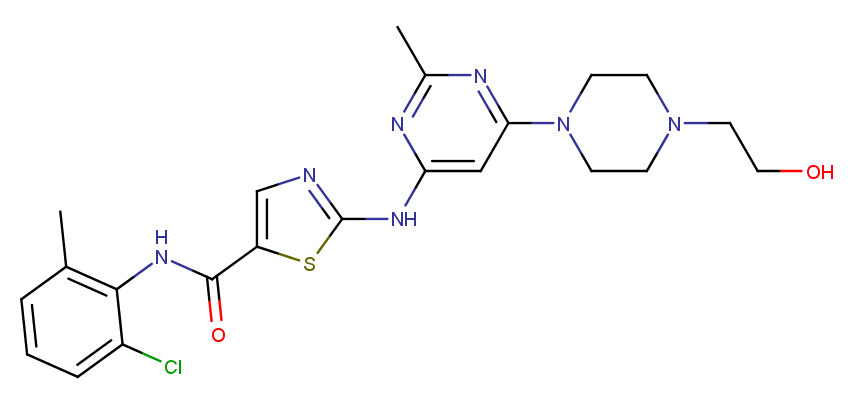
Dasatinib
Enamine offers several subsets of Amino Azole Building Blocks including Aminopyrazoles, Aminothiazoles, Amino?1,2,4-Triazoles and other.. Procedures for the synthesis of these heterocycles were elaborated, and their scope and limitations were established. The methods allow obtaining a large diversity of amino azole building blocks at 1–10 g scale; novel compounds of the requested structure can be obtained in 4–8 weeks. Scale-up to 1 kg can be performed. Moreover, Enamine chemists are experienced in amino azole cyclization reactions with various bis-electrofiles. A large diversity of fused heterocycles having amino azole fragment in their structure can be synthesized upon request.
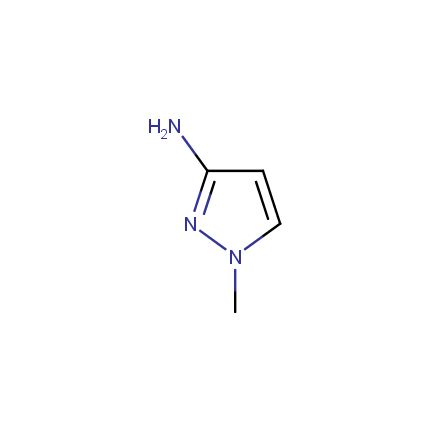
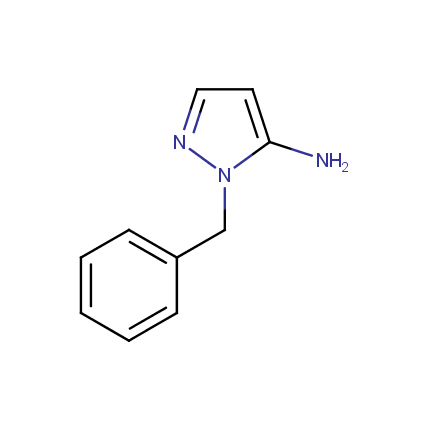
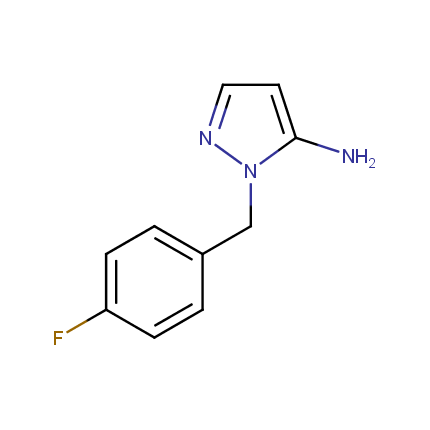
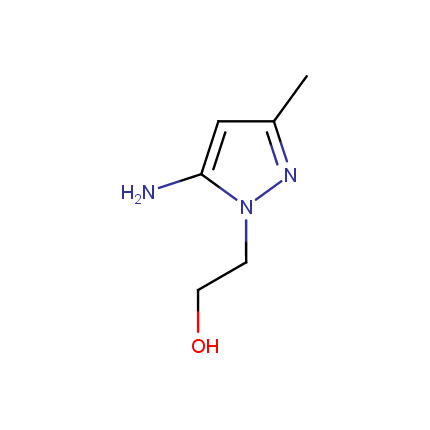
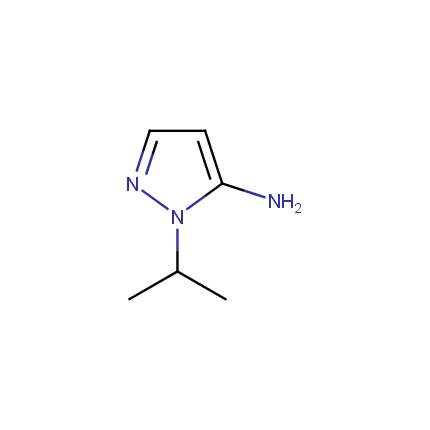
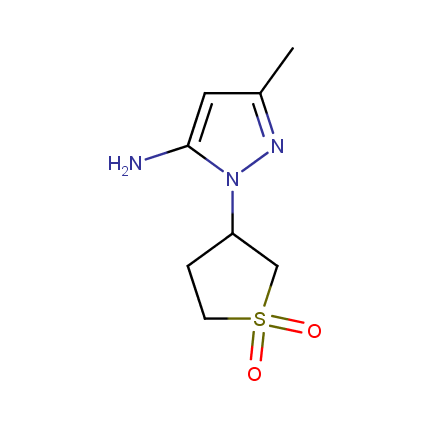
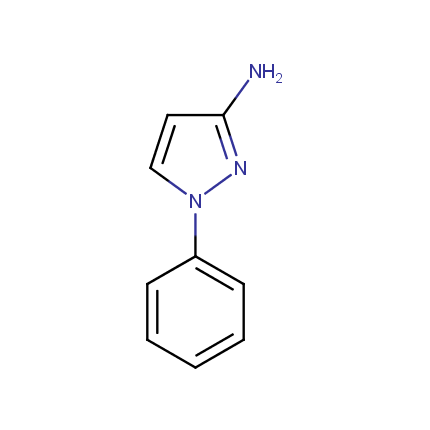
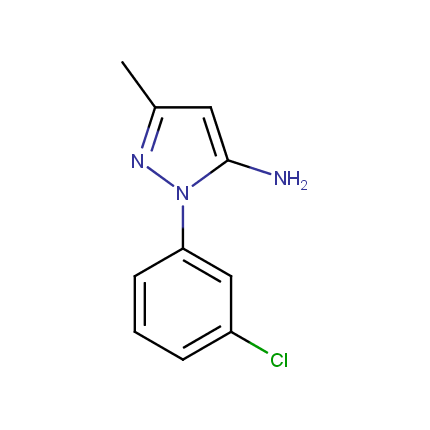
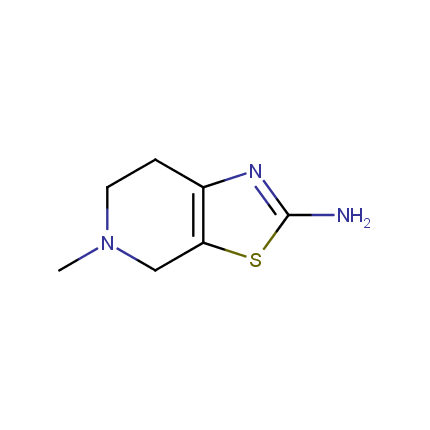
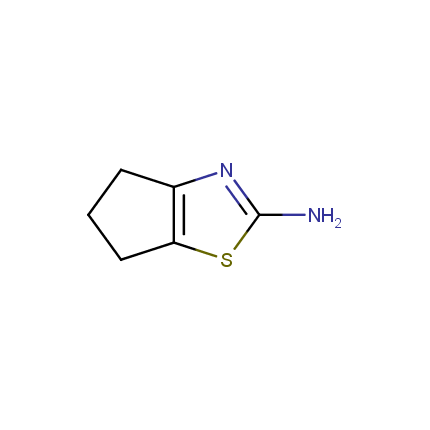
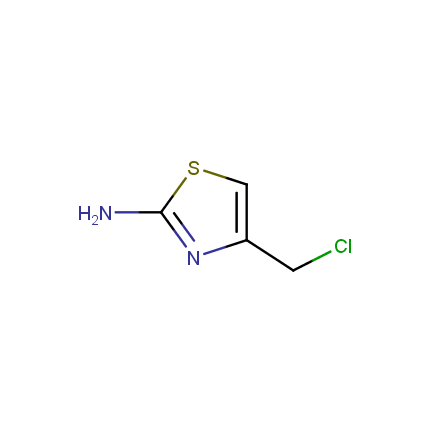
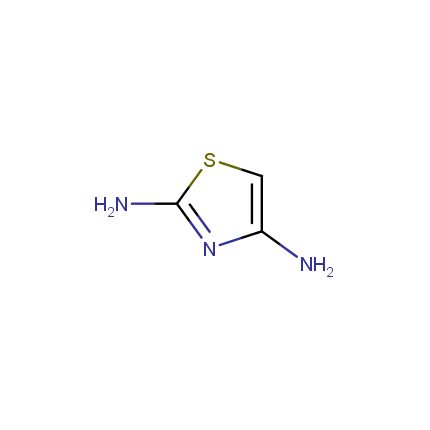
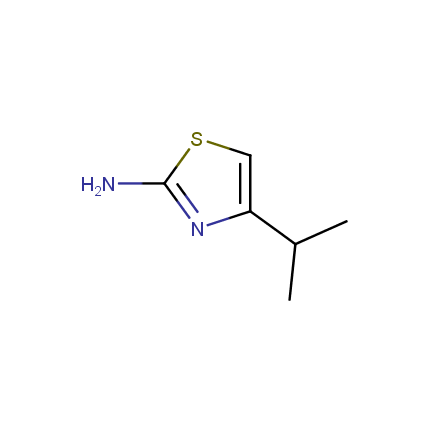
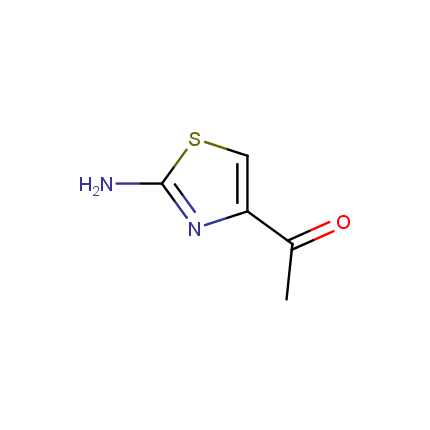
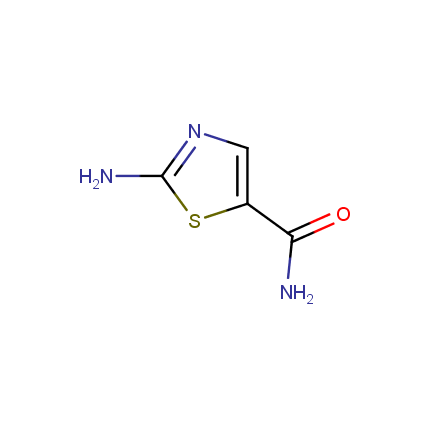
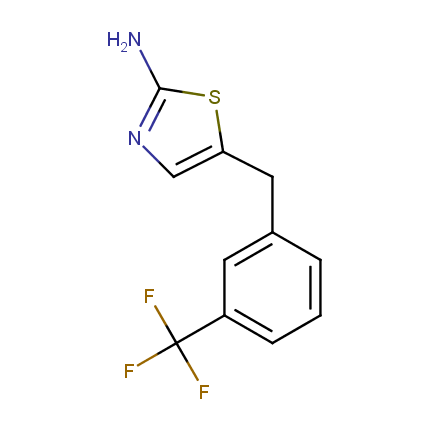
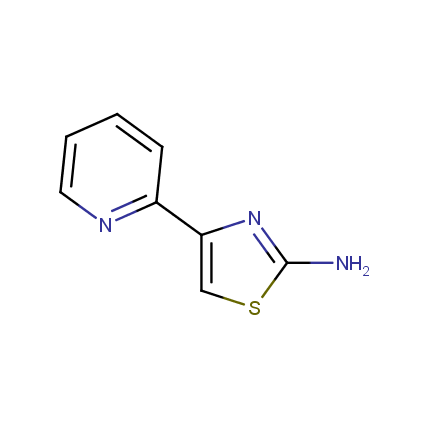
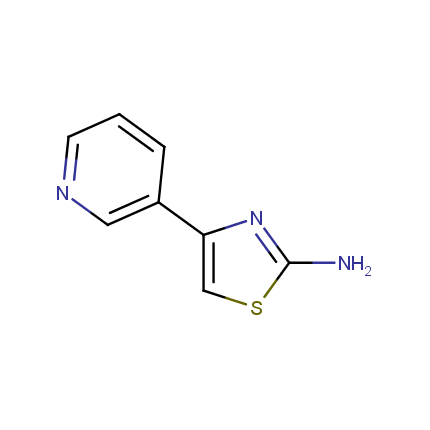
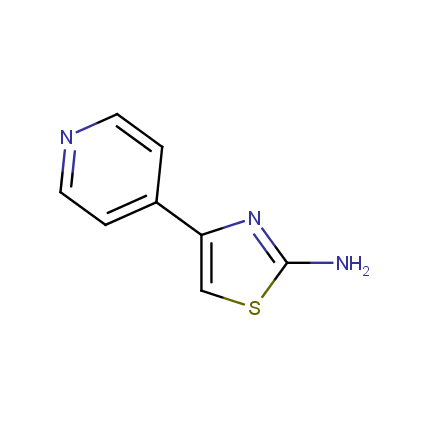
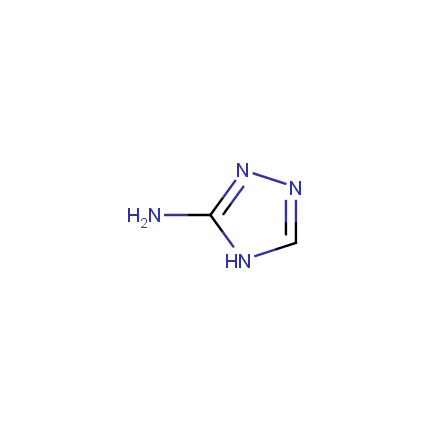
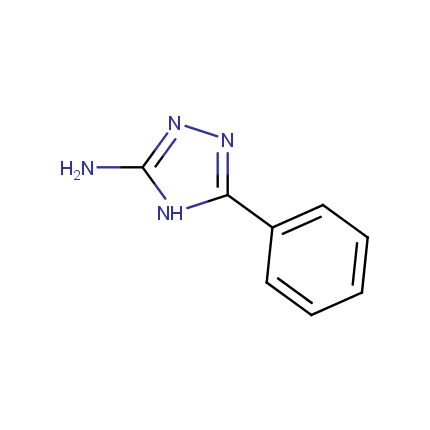
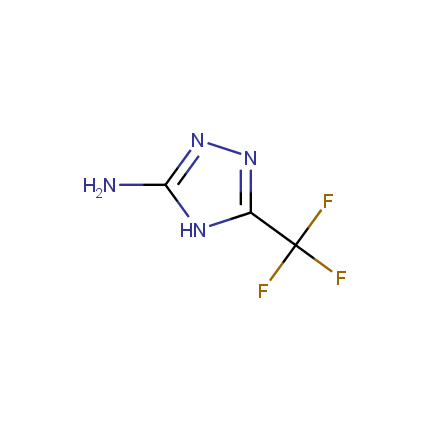
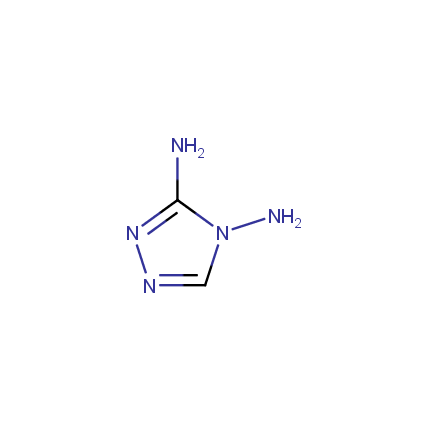
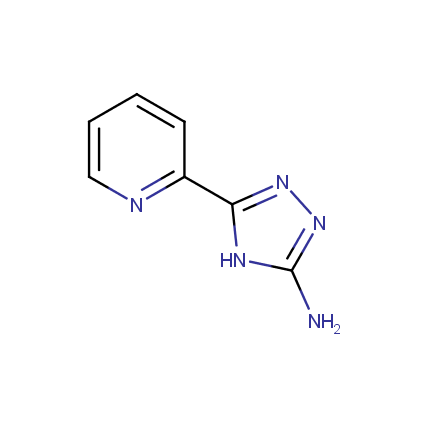
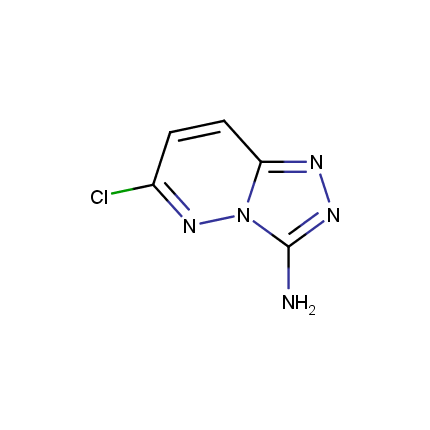
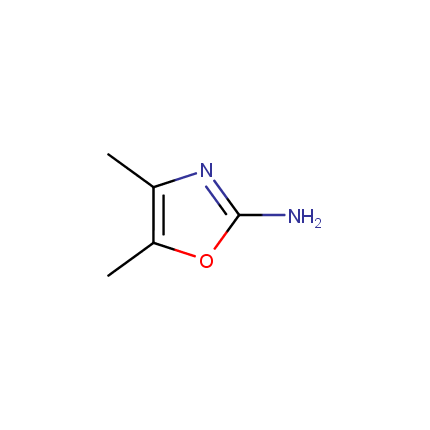
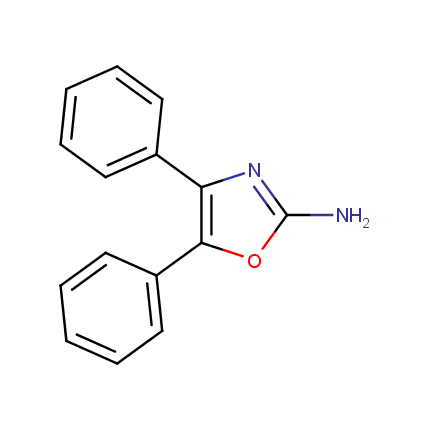
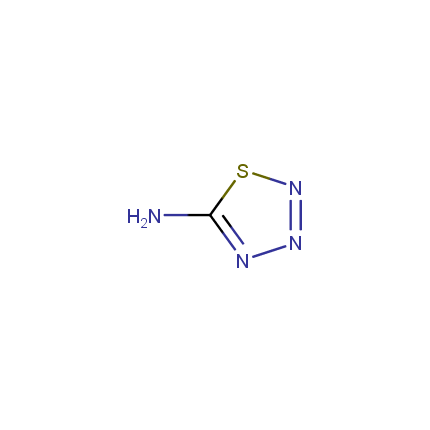
Some examples of Amino Azole Building Blocks are shown below.
Aminopyrazoles
Aminothiazoles
Amino-1,2,4-Triazoles
Other Amino Azoles