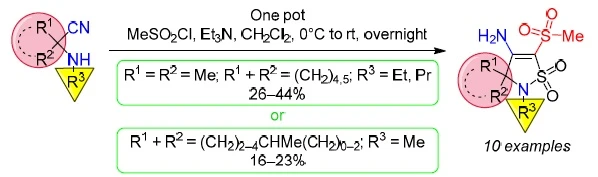
An unexpected synthesis of β-amino-α-mesyl-γ-sultams upon mesylation of hindered α-aminonitriles
Chem. Heterocycl. Compd. 2020, 56 (3), 386–391
DOI: 10.1007/s10593-020-02671-y
We report one-pot procedure for an unexpected synthesis of previously unknown 4-amino-5-methylsulfonyl-2,3-dihydroisothiazole 1,1-dioxides (also called β-amino-α-mesyl-γ-sultams). Thus, the reaction of mesyl chloride with hindered α-aminonitriles is accompanied by the formation of interim N-(1-cyanoalkyl)(methylsulfonyl)methanesulfonamides which, in turn, undergo the сarbanion-mediated sulfonate (sulfonamido) intramolecular cyclization reaction yielding β-amino-α-mesyl-γ-sultams. The structure of the title compounds was confirmed by X-ray diffraction study. The proposed mechanistic insights of the reaction are in accordance with the results of additional experiments and literature data.