Baylis-Hillman Reactions of 2-(Trifluoroacetyl)-1,3-azoles
Synthesis , 2008, 20, 3245-3252
DOI: 10.1055/s-0028-1083150
Khodakovskiy P. V.; Volochnyuk D. M.; Shivanyuk A.; Shishkin O. V.; Tolmachev A. A.
2-(Trifluoroacetyl)-1,3-azoles readily react with methyl acrylate and acrylonitrile under Baylis-Hillman reaction conditions to afford heterocyclic trifluoromethyl-containing allylic alcohols in 36-97% yields. The thus obtained Baylis-Hillman adducts readily undergo Michael addition reactions with various nucleophiles.
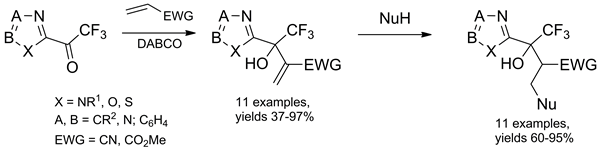
Khodakovskiy P. V.; Volochnyuk D. M.; Shivanyuk A.; Shishkin O. V.; Tolmachev A. A.
Synthesis 2008, 20, 3245-3252
DOI: 10.1055/s-0028-1083150
