Synthesis of Trifluoromethyl-Substituted Proline Analogues as 19F NMR Labels for Peptides in the Polyproline II Conformation
Angew. Chem. Int. Ed. , 2008, 47 (31), 5765-5767
DOI: 10.1002/anie.200801022
Mykhailiuk P. K.; Afonin S.; Palamarchuk G. V.; Shishkin O. V.; Ulrich A. S.; Komarov I. V.
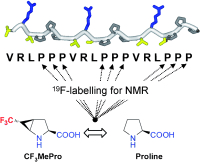
Another CF3-substituted amino acid, CF3MePro (see structure), has been added to the arsenal of 19F NMR labels; it is particularly suited for the study of proline-rich peptides. This amino acid was carefully designed and chosen from other synthesized isomers, according to strict selection criteria, as the most stable, nonracemizable, conformationally restricted, and compatible with solid-phase peptide-synthesis protocols.
Mykhailiuk P. K.; Afonin S.; Palamarchuk G. V.; Shishkin O. V.; Ulrich A. S.; Komarov I. V.
Angew. Chem. Int. Ed. 2008, 47 (31), 5765-5767
DOI: 10.1002/anie.200801022
