Reactions of β-aminovinyl bromodifluoromethyl ketones with alkyl phosphites: Perkow versus Arbuzov
Collect. Czech. Chem. Commun. , 2009, 74 (2), 335-346
DOI: 10.1135/cccc2008095
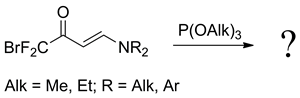
New bromodifluoromethyl enaminones 1a-1f and γ-bromo-β- morpholinopropenyl fluoro-methyl ketones 2a, 2b were synthesized. N-Substituted bromodifluoromethyl enaminones 1a-1d do not react with triethyl or diethyl phosphites, whereas N-acylated enaminones 1e, 1f gave difluorodienyl phosphates 4a, 4b as Perkow rearrangement products. Fluoroketone 2a reacts easily with triethyl phosphite according to the Arbuzov protocol and a perspective building block - trifluoromethyl-containing phosphonate 7a is formed.
Tarasenko K. V.; Gerus I. I.; Kukhar V. P.; Polovinko V. V.
Collect. Czech. Chem. Commun. 2009, 74 (2), 335-346
DOI: 10.1135/cccc2008095
